PIPELINE
TU7710
(A Novel, Recombinant Protein for the Treatment of Hemophilia with Inhibitors)
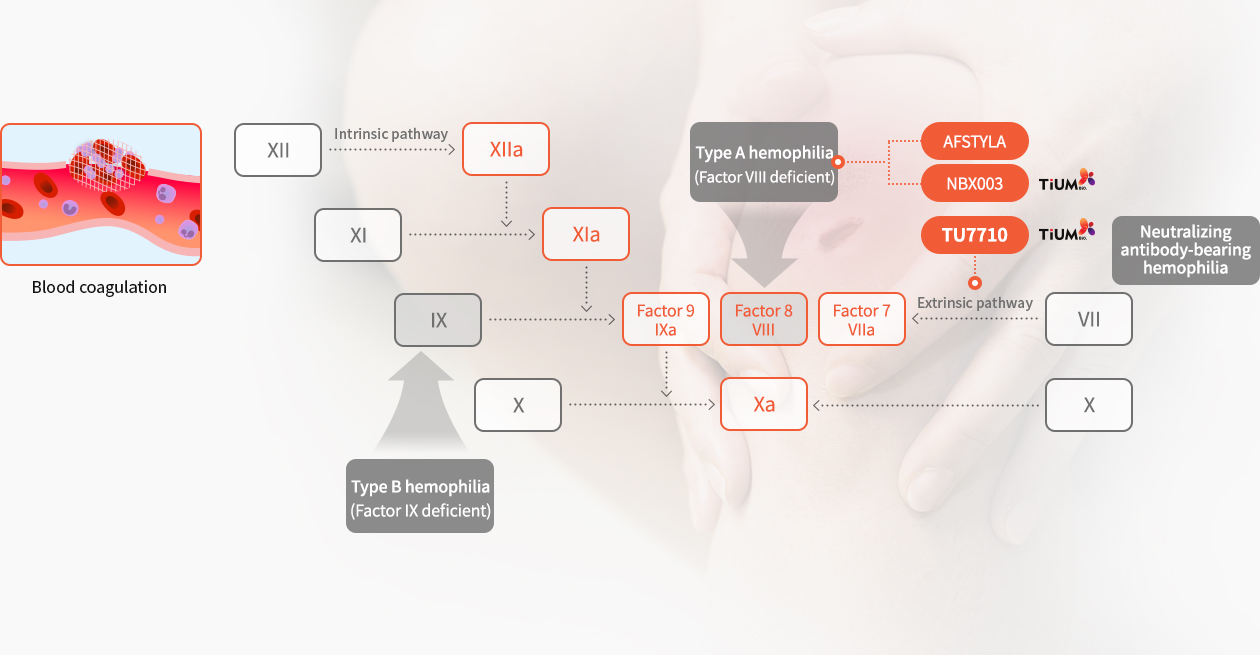
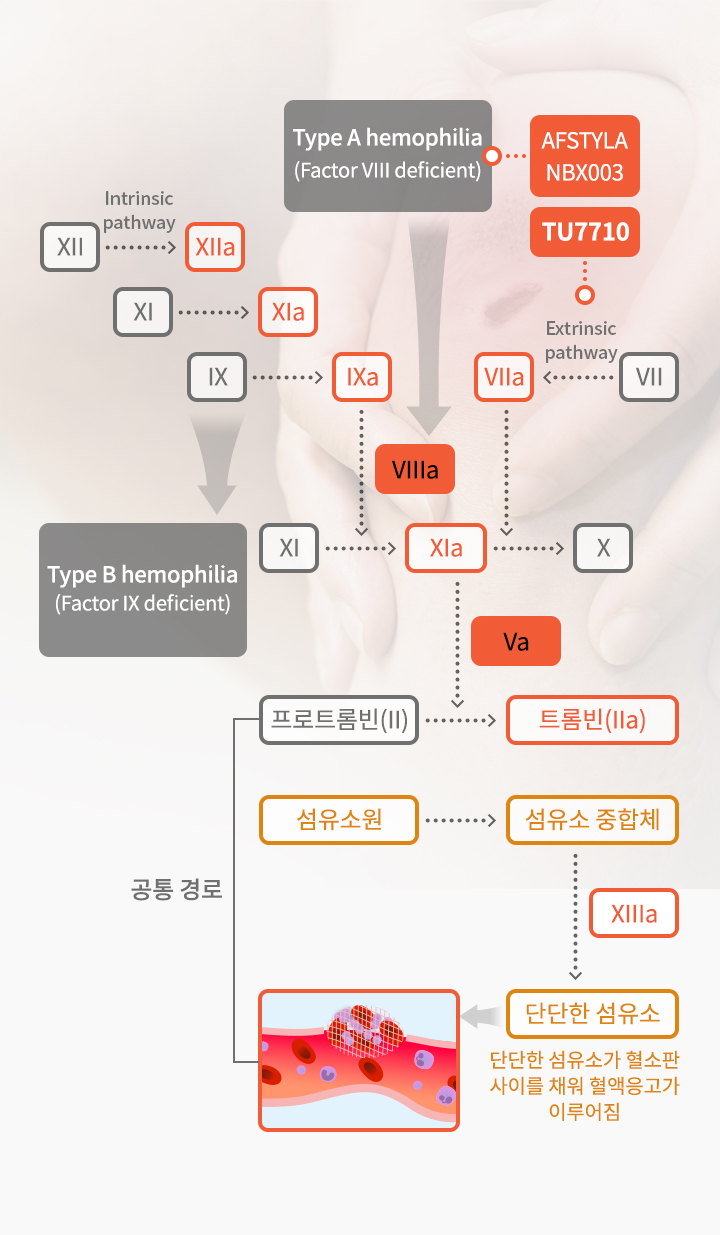
Hemophilia is a rare, inherited bleeding disorder in which the blood does not clot properly due to the lack of sufficient congenital coagulation factors. Hemophilia is a rare disease that occurs about one in every 10,000 people and is classified as Type A (FVIII) and Type B (FIX) depending on the deficiency of each factor, and Type A hemophilia accounts for 80% and Type B hemophilia accounts for 20% of the total. In addition, about 20% of hemophilia patients with type A and type B cannot achieve blood coagulation with conventional treatments due to the occurrence of neutralizing antibodies to hemophilia drugs, so active FVIII is being used as a bypass factor to treat them. As hemophilia is a disease that requires lifelong care after birth, and the main focus of product development is to improve the patient's convenience by increasing the half-life of the drug.
In particular, in the field of bypassing agent drug for hemophilia patients with neutralizing antibodies, it is difficult to use gene therapy, and NovoSeven, which has a short half-life, is exclusively sold, so there is a lot of unmet demand. The market size of bypassing agent including NovoSeven for hemophilia treatment is estimated at $1.5 billion in 2022.
TU7710 is a bypassing agent treatment for patients with neutralizing antibodies and is a innovative drug candidate with a half-life 6~7 times greater than conventional treatments through our transferrin fusion gene recombination technology. TU7710 is expected to dramatically improve the convenience and quality of life of hemophilia patients with neutralizing antibodies. Further, hemophilia treatments are statistically highly likely to be commercialized due to a high success rate in the clinical stage and a short clinical period compared to general drugs.
Our key researchers developed AFSTYLA, a hemophilia treatment approved by FDA and EMA. Based on this expertise and experience, we are working to develop treatments that are more innovative and make patients more convenient.
A clinical trial of TU7710 is currently underway in South Korea.
